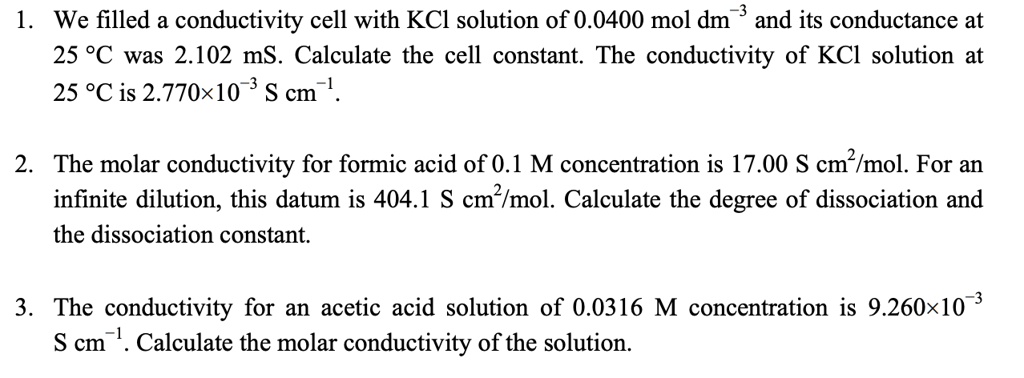
SOLVED: 1 We filled a conductivity cell with KCL solution of 0.0400 mol dm and its conductance at 25 %C was 2.102 mS Calculate the cell constant: The conductivity of KCL solution

How to Find the Thermal Conductivity of a Material Connecting Two Systems Using the Law of Thermal Conduction | Physics | Study.com

The molar conductivity of a 1.5 M solution of an electrolyte is found to be `138.9 S cm^(2) mol^(-1) - YouTube

The conductivity of `0.1`m KCl solution is `1.29sm^(-1)`. If the resistance of the cell filled with - YouTube

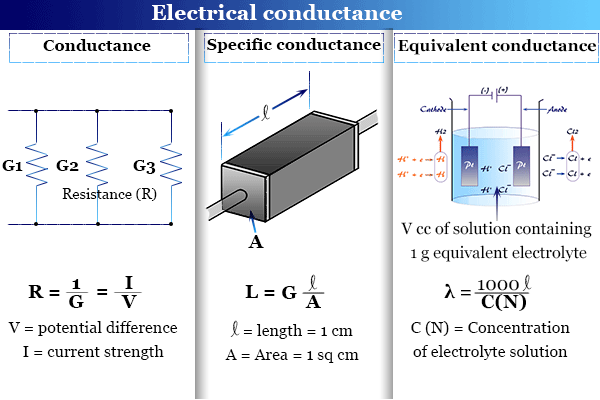
Define the following and write the formula and unit of each:(i) Specific conductivity, (ii) Molar conductivity.

the conductivity of a solution containing 1 04g of anhydrous BaCl2 in 250ml of water has been found to be - Chemistry - Electrochemistry - 13781839 | Meritnation.com
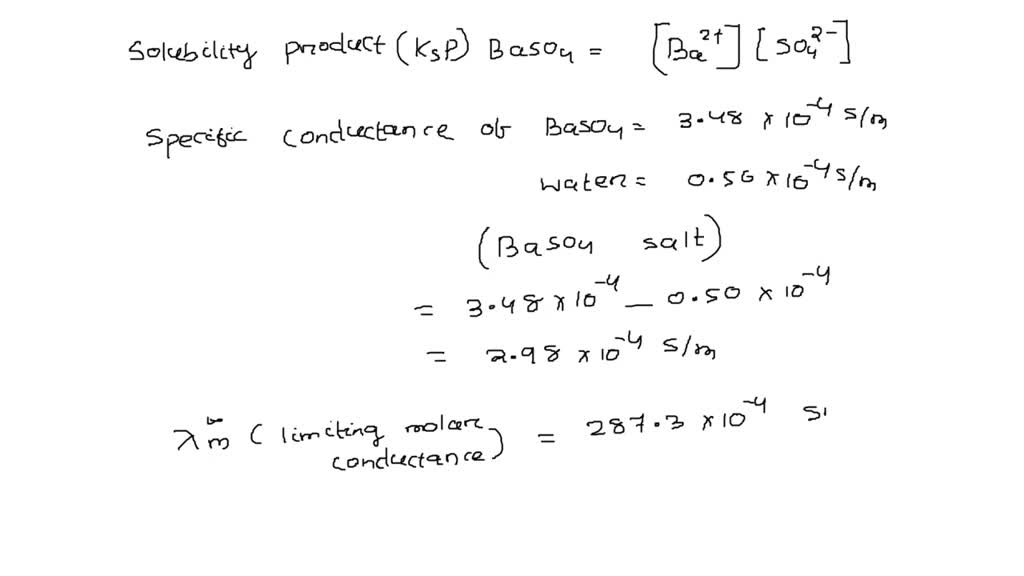
SOLVED: The conductivity of saturated solution of of BaSO4 is 3.48 x 10-4 S/m and the conductivity of pure water is 0.50 x 10-4 S/m at 298 K. Calculate the solubility product

The specific conductivity of a solution containing `1.0g` of anhydrous `BaCI_(2)` in `200 cm^(3)` of - YouTube

Exp 4A: Conductivity Of Aqueous Solutions Purpose –Study conductivity of a series of solutions to determine the difference between strong electrolytes, - ppt download
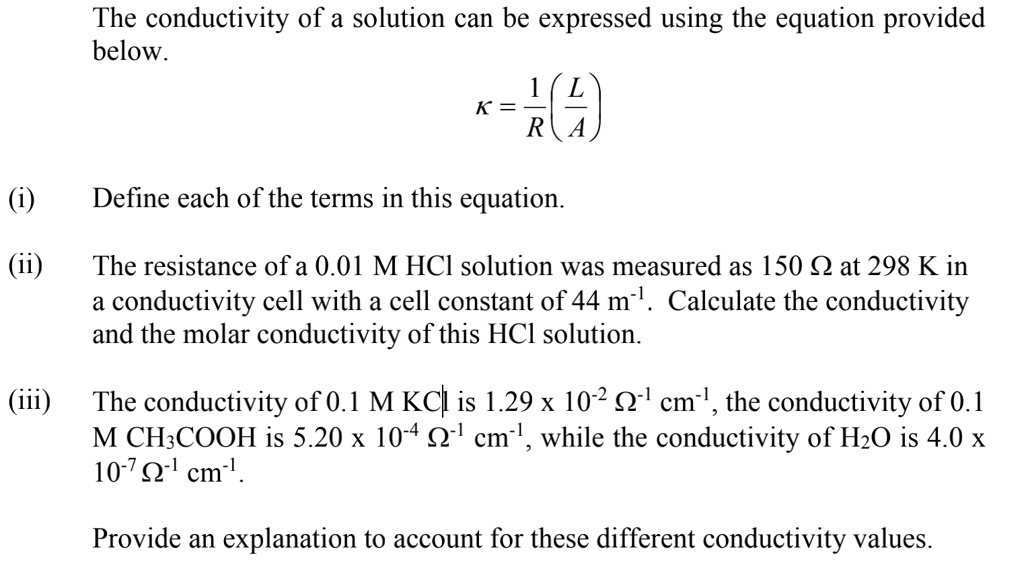
SOLVED: The conductivity of a solution can be expressed using the equation provided below. K = R(4) R Define each of the terms in this equation. (ii) The resistance of a 0.01

What is the Relationship Between Conductance and Resistance? | Series And Parallel Circuits | Electronics Textbook